- Case Report
- Open access
- Published:
Persistent intracranial hyper-inflammation in ruptured cerebral aneurysm after COVID-19: case report and review of the literature
BMC Neurology volume 24, Article number: 17 (2024)
Abstract
Background
The systemic manifestations of coronavirus disease 2019 (COVID-19) include hyperinflammatory reactions in various organs. Recent studies showed evidence for the frequent involvement of central nervous system in affected patients; however, little is known about clinical features of cerebrovascular diseases in childhood-onset COVID-19.
Case presentation
A 10-year-old boy recovered from SARS-CoV-2 infection without complication. On 14 days after infection, he presented with loss of consciousness. A head computed tomography detected a ruptured cerebral aneurysm at the left posterior cerebral artery accompanying subarachnoid hemorrhage (SAH). Immediate surgical intervention did not rescue the patient, resulting in the demise 7 days after admission. Serological and genetic tests excluded the diagnosis of vasculitis and connective tissue disorders. Retrospective analysis showed markedly higher levels of interleukin (IL)-1β, IL-6 and IL-8 in the cerebrospinal fluid than the serum sample concurrently obtained. A review of literature indicated that adult patients with COVID-19 have a risk for the later development of SAH during the convalescent phase of COVID-19.
Conclusions
SAH is a severe complication of COVID-19 in children and adults who have asymptomatic cerebrovascular aneurysms. The markedly high levels of cytokines detected in the cerebrospinal fluid suggested that intracranial hyperinflammatory condition might be one of the possible mechanisms involved in the rupture of a preexisting cerebrovascular aneurysms.
Introduction
The pandemic of COVID-19 continues to grow worldwide. To date, at least 760 million people have contracted severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and 6.8 million deaths have occurred since the first reported case in 2019 [1]. Although initially documented as a respiratory tract-targeted viral infection, SARS-CoV-2 has been associated with inflammatory reactions in systemic organs [2].
Accumulating evidence highlights the neurotropism of SARS-CoV-2, provided that an increasing number of reports show the association with cerebrovascular disease and neuroinflammatory conditions [3]. Hypercoagulability and cytokine release syndrome are also postulated as probable mechanisms of an increased susceptibility to cerebrovasular events in patients with COVID-19 [4].
Cerebrovascular events occur as intracerebral hemorrhage [5] and ischemic stroke [6]. Still rarer complications include cerebral venous sinus thrombosis and aneurysmal subarachnoid hemorrhage (SAH) [7, 8]. SAH might concurrently develop during the acute phase of COVID-19 in adult patients [9], but not reportedly in pediatric patients. We herein present a pediatric patient with ruptured intracerebral aneurysm after COVID-19.
Case description
A 10-year-old Japanese boy entered the emergency room, with complaints of headache and a loss of consciousness in the 2nd week of August, 2022. During this period, the Omicron BA.5 variant of SARS-CoV-2 was the most prevalent strain in Japan. He had no history of hypertension but self-limited infantile epilepsy at age one with normal neuroimaging and electroencephalography results. A computer tomography (CT) image at age one suggested no apparent aneurysmal lesion (Fig. 1A). Fourteen days before admission, he had fever and vomiting with positive test for SARS-CoV-2. After intravenous hydration, he obtained a prompt recovery on the next day. The child had not been previously vaccinated against COVID-19.
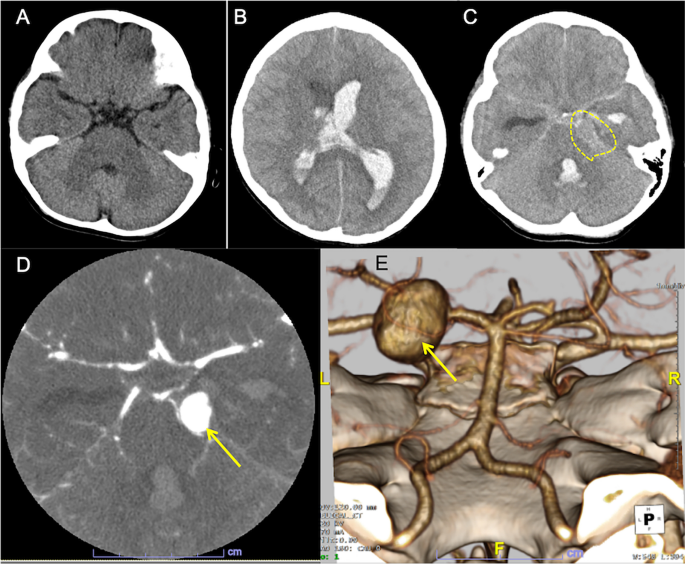
Computed tomography (CT) images. A A CT image taken at age one. No identifiable lesion suggestive of aneurysm. B Axial CT image on admission. The plain CT shows brain swelling, hydrocephalus with intraventricular hemorrhage. C Hyperdense material in the basal cistern, intraventricular hemorrhage in the left lateral and fourth ventricles, as well as a round lesion (encircled) at the left pontocerebellar cistern, indicating intracerebral aneurysm. D CT angiography reveals a large dome-shaped aneurysm (arrow) measuring 15 mm of the left posterior cerebral artery. E 3D-reconstruction image shows a saccular aneurysm in the left P2 segment (arrow)
On admission, left-sided tonic seizure persisted and was terminated after infusions of diazepam. The Glasgow Coma Scale was E2V3M3. A polymerase chain reaction test on the nasopharyngeal sample was negative for SARS-CoV-2. An urgent head CT revealed hydrocephalus with massive intraventricular hemorrhage and the parenchymal edema (Fig. 1B). A hyperdense round-shape lesion was identified at the left pontocerebellar cistern, adjacent to the hyperdense material in the basal cistern (Fig. 1C). These indicated the diagnosis of aneurysmal SAH (WFNS clinical scale: Grade 4). An additional computed tomography angiography (CTA) detected a large saccular aneurysm sized 15 mm of the left posterior cerebral artery (PCA) (Fig. 1D). The 3D-reconstruction image confirmed a large aneurysm in the P2 segment (Fig. 1E).
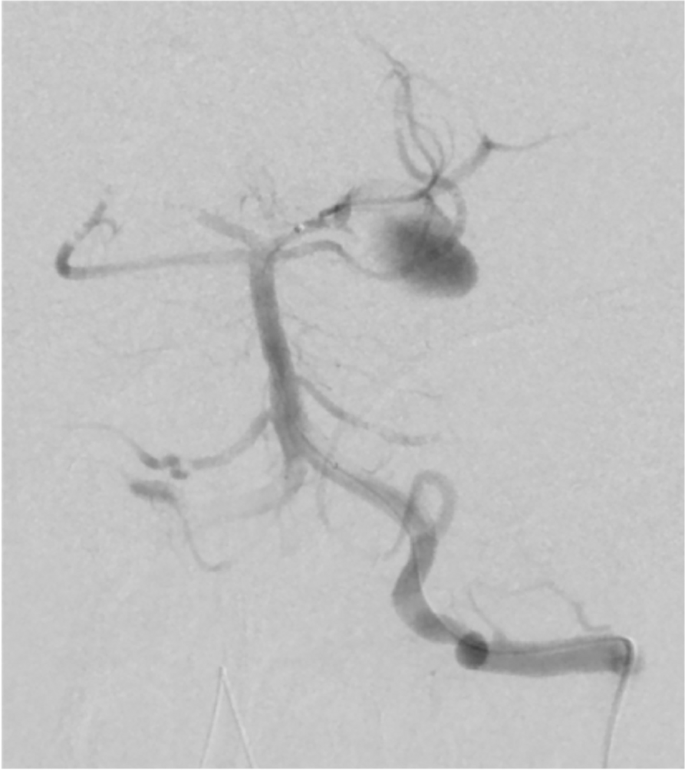
Intensive care was started after immediate bilateral external ventricular drainage. Pupils began to dilatate from Day 2 of admission. The follow-up CT showed progressive brain edema. Left vertebral-angiography revealed poor perfusion to the arteries distal to basilar artery due to the increased intracranial pressure (Fig. 2). Additional interventions with coil occlusion and decompressive craniectomy did not reduce the intracranial pressure to less than 80 mmHg. He died on Day 7th of admission (21 days after the onset of COVID-19). Autopsy had not been performed following caregivers’ refusal to consent.
To exclude vasculitis or vasculopathy, we retrospectively analyzed cerebrospinal fluid (CSF) using “FilmArray” meningitis and encephalitis panel (Biofire Diagnostics, Utah, USA). The test was negative for 14 pathogens, including enterovirus and varicella zoster virus [10]. A genetic diagnostic panel for connective tissue disorders including Ehlers-Danlos syndrome, Marfan syndrome, Loeys-Dietz syndrome reported no pathogenic variants [11]. We measured concurrent cytokine concentrations in serum and the CSF with a flow-cytometric bead assay (BD Biosciences, San Jose, NJ). The CSF sample was obtained one day after the onset of SAH (15 days post-COVID-19) from ventricular drain 24 h after the procedure without evidence of rebleeding. Interleukin (IL)-6 level was > 750 times higher in the CSF showing 273,680 pg/mL compared to concurrently obtained serum sample measuring 356 pg/mL. CSF levels of IL-1β (CSF: 352 pg/mL; serum: 0 pg/mL) and IL-8 (CSF: 310,360 pg/mL; serum: 2,638 pg/mL) were also predominantly elevated. Tumor necrosis factor-α was undetectable in CSF or serum samples.
We reviewed literature on aneurysmal SAH and COVID-19 up to September 2022 (PUBMED search for the keywords: SAH, COVID-19 and aneurysm). We identified a total of 22 cases from 10 articles [7,8,9, 12,13,14,15,16,17,18]. The clinical information is summarized in Table 1. All but one adolescent case [12] were adult patients. The present patient was the youngest of all reported cases. The severity of COVID-19 was varied, ranging from asymptomatic to respiratory distress with systemic involvement (“Severe”). Only one patient had a previously detected aneurysm [17]. Twenty of 23 cases (87%) were SARS-CoV-2-positive during the acute stage of SAH. The remaining 3 (13%) including ours suffered from the later-onset SAH (> 2 weeks after infection). Aneurysmal size ranged from 1.4–21 mm (mean: 8.7 mm). The only one patient (Ref9-10) with PCA aneurysm showed a large aneurysmal size (21 mm) [9], as observed in our case.
Discussion
We report a pediatric patient with SAH resulting from a ruptured aneurysm at PCA 14 days after the recovery of COVID-19. Irrespective of geographical location, the incidence of aneurysmal SAH increased with age, and the mean age at onset was 47.4 years [19]. PCA-involving SAH accounts for 1% of all intracranial aneurysms [20]. Two combined unusual conditions discriminate this case from previously reported cases, suggesting a different patho-mechanism involved. The exclusion of other etiologies of SAH implied the association with a recent COVID-19 infection in this patient.
Cumulative evidence suggest COVID-19 predisposes patients to cerebrovascular events. SARS-CoV-2 virion established infection by binding to the angiotensin-converting enzyme 2 (ACE2), an enzyme critical for regulation of blood pressure and anti-atherosclerotic effects [21]. This may result in increased blood pressure, accelerating cerebral aneurysm formation in a short period. Additionally, COVID-19 induced endothelial damage activates the coagulation cascade [22], which may subsequently contribute to the rapid enlargement of aneurysms and consequently to their ruptures. The hyperinflammatory state in COVID-19 potentially exaggerates the increased permeability of the blood–brain barrier [15], causing elevations in matrix metalloproteinase-9 and subsequent arterial instability.
Despite a number of probable patho-mechanisms thus far proposed, the current knowledge concerning the connection between COVID-19 and SAH is limited in the real-world setting [23, 24]. In fact, there are conflicting data showing the incidence of SAH in patients with COVID-19. For example, a single institute reported that the incidence of SAH and intracerebral hemorrhage was increased during the pandemic period in 2020, compared to that in the reference period one year before [23]. Another study using the multivariate analysis did not find such an increase in the incidence, but did a higher mortality in patients with COVID-19 and SAH [24]. In our patient, the IL-6 level in CSF was not only increased to > 10-fold higher than those in patients with SAH [25], but also to > 100 folds of those with neuro-COVID [26]. Of note, the IL-6 level in CSF was much higher (> 750 times) than serum levels, suggesting that persistent intracranial inflammation raised a risk for the rupture of a preexisting aneurysm after COVID-19.
Childhood-onset SAH in a post-COVID-19 condition might be more than a coincidental finding, given unusual features in classically defined cases of SAH. Although the substantial impact of COVID-19 on the cerebral vasculature remains unknown, neurotropism and intracranial inflammation together with systemic vasculitis might additively contribute to the cerebrovascular vulnerability. Given that this is the first report showing the markedly increased levels of proinflammatory cytokines in CSF from a patient with post-COVID-19 SAH, further epidemiological and clinical studies are needed to rationalize the pathogenic link between COVID-19 and SAH in childhood.
Availability of data and materials
All datasets for this study are included in the manuscript.
References
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 27 March 2023.
Elrobaa IH, New KJ. COVID-19: pulmonary and extra pulmonary manifestations. Front Public Health. 2021;9:711616.
LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–47.
Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16-27.e1.
Margos NP, Meintanopoulos AS, Filioglou D, Ellul J. Intracerebral hemorrhage in COVID-19: a narrative review. J Clin Neurosci. 2021;89:271–8.
Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–49.
Fiani B, Fowler JB, Figueras RA, Hessamian K, Mercado N, Vukcevich O, et al. Ruptured cerebral aneurysms in COVID-19 patients: a review of literature with case examples. Surg Neurol Int. 2021;12:187.
Khan D, Naderi S, Ahmadi M, Ghorbani A, Cornelius JF, Hänggi D, et al. Intracranial aneurysm rupture after SARS-CoV2 infection: case report and review of literature. Pathogens. 2022;11(6):617.
Dodd WS, Jabbour PM, Sweid A, Tjoumakaris S, Gooch MR, Al Saiegh F, et al. Aneurysmal subarachnoid hemorrhage in patients with coronavirus disease 2019 (COVID-19): a case series. World Neurosurg. 2021;153:e259–64.
Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S, et al. Multicenter evaluation of BioFire FilmArray Meningitis/Encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016;54:2251–61.
Ieda D, Hori I, Nakamura Y, Ohshita H, Negishi Y, Shinohara T, et al. A novel truncating mutation in FLNA causes periventricular nodular heterotopia, Ehlers-Danlos-like collagenopathy and macrothrombocytopenia. Brain Dev. 2018;40:489–92.
Savić D, Alsheikh TM, Alhaj AK, Lazovic L, Alsarraf L, Bosnjakovic P, et al. Ruptured cerebral pseudoaneurysm in an adolescent as an early onset of COVID-19 infection: case report. Acta Neurochir (Wien). 2020;162:2725–9.
Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI, Gooch MR, et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15:733–42.
Muhammad S, Petridis A, Cornelius JF, Hänggi D. Letter to editor: severe brain haemorrhage and concomitant COVID-19 Infection: a neurovascular complication of COVID-19. Brain Behav Immun. 2020;87:150–1.
Cezar-Junior AB, Faquini IV, Silva JLJ, de Carvalho Junior EV, Lemos L, Freire Filho JBM, et al. Subarachnoid hemorrhage and COVID-19: association or coincidence? Medicine. 2020;99:e23862.
Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91:846–8.
Rustemi O, Raneri F, Iannucci G, Volpin L, Segna A. Aneurysmal subarachnoid hemorrhage in a SARS-CoV-2 positive testing: casual or causal? Br J Neurosurg. 2022;36:293–4.
Sato T, Miura Y, Yasuda R, Toma N, Suzuki H. Vertebral artery dissecting aneurysm rupture under severe COVID-19. Brain Hemorrhages. 2022;3:210–3.
Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76:588–97.
Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR Am J Neuroradiol. 2001;22:27–34.
Panther EJ, Lucke-Wold B. Subarachnoid hemorrhage: management considerations for COVID-19. Explor Neuroprotective Ther. 2022;2:65–73.
Cañas CA, Cañas F, Bautista-Vargas M, Bonilla-Abadía F. Role of tissue factor in the pathogenesis of COVID-19 and the possible ways to inhibit it. Clin Appl Thromb Hemost. 2021;27:10760296211003983.
Kafadar S, Yucetas SC, Gezgin I, Kaya H, Gulacti U, Bilek O. Influence of COVID-19 disease on subarrachnoid hemorrhage and intracerebral hemorrhage. Bratisl Lek Listy. 2022;123:140–3.
Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Labanova I, et al. Subarachnoid hemorrhage and COVID-19: an analysis of 282,718 patients. World Neurosurg. 2021;151:e615–20.
Ridwan S, Grote A, Simon M. Interleukin 6 in cerebrospinal fluid is a biomarker for delayed cerebral ischemia (DCI) related infarctions after aneurysmal subarachnoid hemorrhage. Sci Rep. 2021;11:12.
Jarius S, Pache F, Kortvelyessy P, Jelcic I, Stettner M, Franciotta D, et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation. 2022;19:19.
Acknowledgements
We thank the patient’s family for their participation.
Funding
This work was supported in part by JSPS KAKENHI grant number JP19K10613 (Chong); research grants from the Ministry of Health, Labour and Welfare of Japan (grant numbers JP22HA1003 to Chong, and JP20FC1054 to Sakai); AMED under grant numbers JP20ek0109411, JP20wm0325002h; The Japan Epilepsy Research Foundation, and Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics (Sakai).
Author information
Authors and Affiliations
Contributions
Chong, Sakai, and Ohga conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Higashi, Matsuoka, Sangatsuda, Iwaki, Sonoda, Ichimiya, Kamori, Kawakami, and Mizuguchi provided care for the patient, performed the initial analyses and reviewed the manuscript. Arimura and Kaku supervised data collection, and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the ethics committee of Kyushu University Hospital (number 22258-00).
Consent for publication
Written informed consent to participate in this study was provided by the participant’s legal guardian.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chong, P.F., Higashi, K., Matsuoka, W. et al. Persistent intracranial hyper-inflammation in ruptured cerebral aneurysm after COVID-19: case report and review of the literature. BMC Neurol 24, 17 (2024). https://doi.org/10.1186/s12883-023-03493-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03493-z