- Research
- Open access
- Published:
Neuromuscular diseases associated with COVID-19 vaccines: a systematic review and pooled analysis of 258 patients
BMC Neurology volume 23, Article number: 437 (2023)
Abstract
Background
Neuromuscular diseases (NMD) emerged as one of the main side effects of the COVID-19 vaccination. We pooled and summarized the evidence on the clinical features and outcomes of NMD associated with COVID-19 vaccination.
Methods
We comprehensively searched three databases, Medline, Embase, and Scopus, using the key terms covering “Neuromuscular disease” AND “COVID-19 vaccine”, and pooled the individual patient data extracted from the included studies.
Results
A total of 258 NMD cases following COVID-19 have been reported globally, of which 171 cases were Guillain-Barré syndrome (GBS), 40 Parsonage-Turner syndrome (PTS), 22 Myasthenia Gravis (MG), 19 facial nerve palsy (FNP), 5 single fiber neuropathy, and 1 Tolosa-Hunt syndrome. All (100%) SFN patients and 58% of FNP patients were female; in the remaining NMDs, patients were predominantly male, including MG (82%), GBS (63%), and PTS (62.5%).
The median time from vaccine to symptom was less than 2 weeks in all groups. Symptoms mainly appeared following the first dose of vector vaccine, but there was no specific pattern for mRNA-based.
Conclusion
COVID-19 vaccines might induce some NMDs, mainly in adults. The age distribution and gender characteristics of affected patients may differ based on the NMD type. About two-thirds of the cases probably occur less than 2 weeks after vaccination.
Highlights
• Neuromuscular diseases (NMD) have emerged as a significant side effect of COVID-19 vaccination.
• The study provides a comprehensive summary of the clinical features and outcomes of NMD associated with COVID-19 vaccination.
• A total of 258 cases of NMD following COVID-19 vaccination were reported globally.
• The most commonly reported NMDs were Guillain-Barré syndrome (GBS) and Parsonage-Turner syndrome (PTS).
• Symptoms typically appeared within 2 weeks after vaccination, with a higher likelihood following the first dose of vector vaccine.
Introduction
In the long term, vaccination remains vital in the COVID-19 pandemic control strategy [1]. More than 13.3 billion COVID-19 vaccines have been administered globally; however, the fading of immunity over time necessitates booster doses; hence, about 1 million shots are still administered daily worldwide [2].
However, the extraordinary speed of vaccine production and the waiver of essential testing steps raised hesitancy regarding the vaccines’ safety [3,4,5]. A wide range of side effects are reported worldwide shortly after vaccination. While most side effects of vaccines are mild, their impact on the central and peripheral nervous systems often necessitates medical interventions [6,7,8,9,10].
Recently, increasing case series/reports have characterized the clinical course in patients with some types of peripheral neuropathy (Guillain-Barré syndrome, Parsonage-Turner syndrome, Small fiber neuropathy, and Tolosa-Hunt syndrome), cranial neuropathy (Facial nerve palsy), and neuromuscular junction disorder (Myasthenia gravis) [11] following COVID-19 vaccination. In this study, we pooled and summarized available evidence to enhance the knowledge of the course and prognosis of the neuromuscular diseases associated with COVID-19 vaccines, which can lead to a well-timed diagnosis and management of these patients.
Method
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Patient consent or ethical approval was not required due to the review nature of the study. All studies reporting the symptoms and clinical course of patients with neuromuscular disease (NMD) associated with the COVID-19 vaccine were included.
Search
We comprehensively searched three databases, Scopus, Medline, and Embase, on 25 January 2023 and updated on 20 February 2023, using a combination of the key terms in multiple domains, including “COVID-19 vaccine”, “Peripheral neuropathies”, “Cranial neuropathy”, “neuromuscular disease”. Also, we checked the reference lists and citing publications of the included articles for any additional eligible studies. We imported all retrieved citations into Zotero 6.0.20 and removed the duplicate items.
Inclusion process and criteria
Two researchers [AT and PS] independently screened the titles, abstracts, and full texts of the studies to include the eligible articles. All studies meeting our eligibility criteria were included:
-
1.
Reporting new-onset case (s) of any NMD following COVID-19 vaccination
-
2.
An observational design: case series, case reports, and cohort studies
-
3.
Written in English
Data extraction
Three researchers [EJ, SM, NM] extracted the data from the retrieved articles into a predefined form in Microsoft Excel (version 2019, Microsoft Corp., Redmond, WA, USA). The extracted data included the first author’s name, year, country, age, gender, clinical data, type of the NMD, subtype of the disease, vaccine type, vaccine dose number (first, second, booster), the time between injection and symptom onset, presenting signs and symptoms, electrophysiological findings, laboratory data, treatment, and patients follow up.
Quality assessment
The quality of the included case series was assessed independently by two trained researchers (AA and SM) using the Joanna Briggs Institute (JBI) appraisal tool adapted for case series [12]; a third researcher (AT or HR) resolved any disagreement. JBI appraisal tool included 10 items, each receiving one score (Supplementary Table 1).
Statistical analyses
To organize the extracted individual patient data (IPD) and to produce tables, we used Microsoft Excel (version 2019, Microsoft Corp., Redmond, WA, USA). Based on the disease, we categorized integrated IPD case series and case reports into a relevant case series. After excluding cases without data for the characteristic of interest, we calculated a valid percent for each categorical and a median (Interquartile range) or mean and standard deviation (SD) for continuous variables. Data analysis was conducted using Stata/MP Version 16 (Stata Corp. LP, USA/ METAN package).
Results
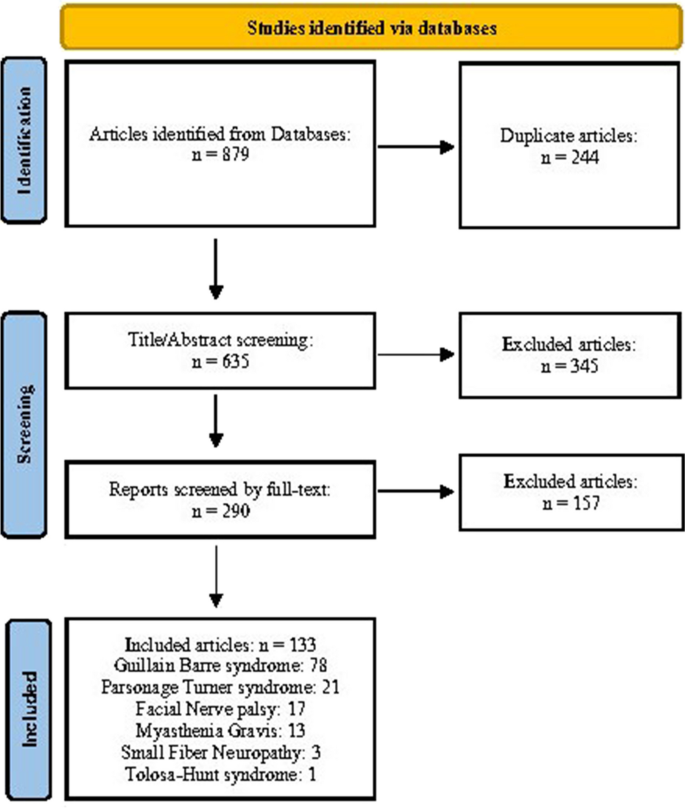
After excluding duplicates (n = 244) and ineligible items from the 879 publications identified in the initial search, 133 case reports/case series met our eligibility criteria. All included studies had a quality score greater than 7/10 (Supplementary Table 1).
Based on our pooled analyses, a total of 258 cases of NMD associated with COVID-19 vaccines have been reported globally. In order by frequency, the type of NMD was Guillain-Barré syndrome (GBS, n = 171), Parsonage-Turner syndrome (PTS, n = 40), Myasthenia gravis (MG, n = 22), Facial nerve palsy (FNP, n = 19), Small fiber neuropathy (SFN, n = 5), and Tolosa-Hunt syndrome (n = 1) (Fig. 1). Reported cases were mainly adults (98%) aged 18 years or older, and in more than two-thirds of them, the symptoms appeared less than 2 weeks after the vaccination.
Guillain-Barré syndrome
Seventy-eight articles (57 case reports [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] and 21 case series [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90]) containing 171 COVID-19 vaccine-associated GBS patients were reported. About half of the cases were reported from South Korea (n = 21), Italy (n = 20), The USA (n = 18), and Australia (n = 18) (Supplementary Tables 2 and 3).
The reported cases were aged between 14 and 90 years old, with a median (IQR) of 58 [43, 70] years, and 108 (63%) of cases were male. Regarding vaccine type, 31% (52/168) of patients received an mRNA and 69% (116/168) a vector-based vaccine. In most cases vaccinated with vector-based vaccines, symptoms appeared after the first dose (First/ Second doses (n): 105/ 11); however, such a pattern was not observed in cases vaccinated with mRNA-based vaccines (First/ Second/ Booster doses (n): 34/ 16/ 2). The median (IQR) time from vaccine to symptoms was 13 [8, 17] days, ranging from 1 to 44 days. Regarding GBS pathogenic subtypes, 81% (77/95) of patients had acute inflammatory demyelinating polyneuropathy, 12% (11/95) acute motor axonal neuropathy, and 7% (7/95) acute motor and sensory axonal neuropathy. GBS clinical variants and pathogenic subtypes are summarized in Table 1.
Brain MRI was diagnostic for GBS in only 20 out of 69 patients (sensitivity: 29%); however, Electromyogram / Nerve conduction study (EMG / NCS) was diagnostic in almost all cases (131/135, 97%). Regarding laboratory tests, Albuminocytological dissociation and Anti-ganglioside antibodies were positive in 85% (105/123) and 24% (17/70) of patients, respectively. Based on the reported data, most patients were treated with IVIG only (107/142, 75%); 7% (10/142) underwent IVIG plus plasma exchange treatment, and 6% (9/160) received plasma exchange alone (Table 1). At the follow-up, complete or partial recovery was noted for most patients (65/123 (53%) and 46/123 (37%), respectively). However, death occurred in 3 patients (2%), and 11 out of 123 patients (9%) had a poor recovery.
Parsonage-turner syndrome
A total of 40 patients with PTS associated with COVID-19 vaccines [77, 91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110]. Most cases were reported from The USA (n = 15) and South Korea (n = 13) (Table 1, Supplementary Table 4).
The median (IQR) age was 50 [38, 63] years, ranging from 14 to 84 years, and 25 (62.5%) patients were male. Regarding vaccine type, 70% of cases (n = 28) received mRNA-based vaccines. While in most of the patients with vector vaccine-associated PTS, the symptoms appeared on the first dose of the vaccine (10/12), such a pattern was not observed in mRNA vaccine-related PTS (First/Second doses (n): 14/14).
Of the 38 cases reporting the symptoms localizations, 30 (79%) experienced symptoms ipsilaterally on the injection side, 5 contralateral to the injection side, and 3 bilateral. The presenting symptoms were shoulder or arm pain (20/28), muscle weakness (17/28), and paresthesia (8/28). The median (IQR) interval from vaccine injection to symptoms was 8 [5, 15] days, ranging from 1 to 56 days. EMG / NCS was diagnostic in 85% (22/26) of the patients, and brachial plexus MRI in 31% (11/35). The follow-up duration varied among the studies. However, a complete recovery was noted in 41% (15/37) of patients, a partial recovery in 41% (15/37), and a poor improvement in symptoms in 18% (7/37) (Table 1, Supplementary Table 5).
Facial nerve palsy
Nineteen FNP cases were reported following COVID-19 vaccination [111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127] across the world. The patients’ ages ranged from 17 to 62 years (median (IQR): 38 [34, 57] years), 11 out of 19 patients were female (58%), and 4 patients had a prior history of FNP. In 14 patients, the symptoms occurred after receiving mRNA-based (Pfizer or Spikevax) and 5 after vector-based vaccines (AstraZeneca, Covaxin, Sputnik V, or Johnson and Johnson). Symptoms appeared mainly after the first dose in mRNA- and vector-based vaccine-associated FNP patients. Almost all patients had an ipsilateral FNP (17/19). The time interval from vaccine injection to symptoms ranged from 1 to 18 days, with a median (IQR) of 3 [2, 6] days (Table 1, Supplementary Table 6).
Regarding medications, 10 (56%) patients were treated with corticosteroids alone, 6 (33%) with both corticosteroids and a viral agent. Corticosteroids were combined with Fluorometholone eye drops in one case and with a topical antibiotic in another patient. At the follow-up, the symptoms had resolved in all patients without any complications.
Small fiber neuropathy
We included three articles (two case reports [128, 129] and one case series [130]) reporting five COVID-19-associated small fiber neuropathy (SFN) in our study (Table 1, Supplementary Table 7). These patients were reported from Austria (n = 3) and the USA (n = 2). All five patients were female, aged between 32 and 64, and had received mRNA vaccines (Pfizer and Spikevax). In 4 out of 5 patients, symptoms appeared after receiving the second dose.
The presenting symptoms were weakness (2/5), gait disturbances (2/5), dysesthesia (2/5), paresthesia (1/5), and dysphagia (1/5). Skin punch biopsy helped the diagnosis in all cases (4/4). Treatment was reported in 4 patients. Except for patient #1, who was treated with corticosteroid and IVIG, the other patients only received symptomatic therapies. The outcome was reported in 3 of the patients; symptoms resolved in all three in less than 2weeks (Table 1).
Tolosa-hunt syndrome
One Tolosa-Hunt patient from the USA was included in our study [131] (Supplementary Table 7). The patient was a 45-year-old male who received a vector-based vaccine. The patient presented with left-sided headache, periorbital pain, ptosis, and decreased visual acuity. The brain CT indicated a sinus thrombosis, while the brain MRI reported a perineural enhancement surrounding the optic nerve sheath. The patient underwent steroid treatment, and the symptoms improved in 2 months.
Myasthenia gravis
Twenty-two Myasthenia Gravis (MG) patients associated with COVID-19 vaccines were reported from eight countries [8, 132,133,134,135,136,137,138,139,140,141,142,143], more frequently from the UK (n = 7) and Italy (n = 5) (Supplementary Tables 8 and 9).
Patients age ranged from 13 to 90 years (median (IQR): 64 [50, 74] years) and 18 of them were male (18/22). Most patients (14/22) received an mRNA-based vaccine (Pfizer-BioNTech or Spikevax). Vector-based vaccine receivers developed symptoms mainly after the first dose (7/8); however, such a pattern was not observed in patients who received mRNA-based vaccines (First/ second/ third doses (n): 5/ 5/ 4, Table 2).
The presenting symptoms were binocular diplopia (10/13), ptosis (6/13), dysarthria (3/13), dysphagia (3/13), and/or weakness of the lower or upper limbs (1/13). The median (IQR) time interval from vaccine injection to symptoms was 6 (2, 8.5) days. In terms of MG type, 10 patients had an ocular or bulbar MG, and the rest generalized MG (Table 2).
Imaging evaluations were performed for some patients to rule out other causes, including brain CT scan (abnormal findings: 0/5), brain MRI (abnormal findings: 1/6), chest CT scan (abnormal findings: 2/8, Thymoma or thymic hyperplasia) (Table 2, Supplementary Table 9).
Repetitive nerve stimulation (RNS) was positive in 13 out of 15, and Single fiber electromyography (SFEMG) in all 4 patients who received the test. Acetylcholine receptor (AChR) antibody was positive in 16 out of 20 patients and Muscle-specific kinase (MuSK) negative in all 4 tested patients. Complete improvement at discharge/1-month follow-up was reported in 79% (11/14), a myasthenic crisis in 14% (2/14), and unchanged symptoms at 3 months in 7% (1/14) of the cases.
Discussion
Based on our comprehensive literature review, a total of 171 GBS, 40 PTS, 22 MG, 19 FNP, 5 SFN, and 1 Tolosa-Hunt syndrome cases following COVID-19 vaccination have been reported so far across the world. Overall, reported cases were mainly adults; FNP patients (median age: 38 years) were the youngest, and MG patients (64 years) were the oldest group. While all SNF and most FNP cases were female, in other groups, the male gender was predominant. The median time from vaccine to symptom was less than 2 weeks in all groups, ranging from 3 days in FNP patients to 13 days in GBS cases. Symptoms mainly appeared following the first dose of vector-based vaccine, but there was no specific pattern for mRNA-based associated NMD. While most reported cases experienced a benign course of the disease, death occurred in 3 out of 123 GBS patients, and 2 out of 14 MG patients developed a myasthenic crisis.
The occurrence of NMD has been reported following influenza and other vaccines [133, 144,145,146,147,148]. A pooled analysis of GBS following mass immunizations with Influenza, Human papillomavirus, and Measles-Rubella vaccines, including 58 studies (2,110,441,600 participants), revealed an incidence rate of 3.09 per million in 6 weeks of vaccination, without a sex difference. Contrary to GBS induced by COVID-19 vaccines, most GBS cases associated with other vaccines were younger than 18 or older than 60 years old; their different target population could explain this dissimilarity. Furthermore, based on the CDC’s vaccine adverse events reporting system (VAERS) database, 944 individuals developed FNP after other vaccines in the United States between 2009 and 2018 [149]. A little more than half of the patients were female (55.7%), and only 2.2% had a prior FNP history. A similar gender pattern was observed in FNP following COVID-19 vaccines. However, a prior history of FNP was reported in 21% of patients.
Also, a total of 42 definite incident cases of MG occurred following Influenza and Hepatitis B vaccines in American adults between 1990 and 2017 [150]; these cases had a higher mean (SD) time from vaccine to symptoms compared to MG cases associated with COVID-19 vaccines (10 (10.7) vs 7.1(6.6), respectively). Overall, MG is mainly diagnosed between 10 and 70 years old [23, 24], and affected female patients are younger than their male counterparts (mean age: 28 vs. 42 years, respectively) [25]. Gender ratio differs by MG type; contrary to pure ocular or bulbar MG, cases with generalized MG are predominantly female (female/male ratio: 3:2 or higher) [26]. Less than 40% of MG cases following other vaccines were older than 60 years (Mean age (SD): 49.0 (18.9) years, Range: 18 to 84 years), without a gender predominance [22]. However, COVID-19 vaccine-associated MG cases were predominantly male, overall, and by MG type, and half of them were aged 70 years or older (Mean age (SD): 61.0 (19.3) years).
In this study, we summarized data on COVID-19 vaccine-associated NMD provided by case reports/series. The exact mechanism by which vaccines could induce neuromuscular diseases remains unknown. However, the immunization processes following the vaccines could provoke an autoimmune response by establishing an inflammatory environment. If vaccine antigens mimic self-antigens, the immune response could cross-react with self-antigens, leading to an autoimmune reaction (Molecular mimicry mechanism). Furthermore, during immune responses to vaccine antigens, inflammatory signals can activate self-reactive T cells involved in the autoimmune processes (Bystander effect mechanism). Also, the activation of self-reactive T cells could be triggered via self-antigens released upon self-tissue damage following inflammatory cascade (Epitope spreading mechanism). Vaccines containing dsRNA or its analogs can also overexpress IFN-b, a key factor in thymic events leading to MG [3].
The temporal association of neuromuscular disorders and COVID-19 vaccination cannot easily be translated into a causal relationship, and the evidence in this regard is inadequate [151]. Thus, further studies are required to clarify this association.
SFN is categorized as primary and secondary to vaccination or diseases such as kidney failure, diabetes, infections, or autoimmune. As Finsterer et al.129 noted, the clinical presentation of COVID-19 vaccine-associated SFN is similar to other secondary SFNs. Also, evidence on the beneficial effects of IVIG supports the autoimmune nature of the SFN associated with COVID-19 vaccines.
The median time from vaccine to symptom was less than 2 weeks in all NMD, mainly after the first dose; a case can be made that vaccination may have exacerbated an already existing but asymptomatic form in the vaccinated individuals instead of triggering a new onset of the disease.
Limitation and strengths
This review acknowledges certain limitations inherent in our study design. Firstly, due to the nature of the collected data, We couldn’t analyze the link between the number of COVID-19 vaccine doses and neuromuscular disorders. Furthermore, we couldn’t assess the effects of mixing different COVID-19 vaccines for primary and booster doses. Our study can’t conclusively establish a causal relationship between COVID-19 vaccination and neuromuscular events. Few cases of neuromuscular disorders like Tolosa-Hunt syndrome and SFN were reported.
Furthermore, our pooled data sets were incomplete for some variables, such as laboratory tests or imaging. However, we pooled data from all reported cases of NMD following COVID-19 vaccines using a comprehensive search strategy to provide a better understanding of this issue. To our knowledge, this is the first systematic review of reported cases of COVID-19 vaccine-associated NMD.
Conclusion
Based on our comprehensive literature review, COVID-19 vaccines might induce some neuromuscular diseases. These cases mainly occurred after administering the most frequently used COVID-19 vaccines. The age distribution and gender characteristics of affected patients may differ based on the NMD type. About two-thirds of the cases occurred in less than 2 weeks after vaccination. Most cases developed the symptoms following the first dose of vector-based vaccines. The majority of these patients experienced a benign disease course.
Availability of data and materials
All data and materials used in this study are accessible upon request. For inquiries regarding data and materials, please contact the corresponding author.
References
Mariatulqabtiah AR, Buttigieg KR. COVID-19 vaccinations for children. Lancet Infect Dis. 2022;22:1255–6.
Mathieu E, et al. Coronavirus pandemic (COVID-19). Our World Data; 2020.
Zhou Q, Zhou R, Yang H, Yang H. To be or not to be vaccinated: that is a question in myasthenia gravis. Front Immunol. 2021;12:733418.
Samimisedeh P, Jafari Afshar E, Shafiabadi Hassani N, Rastad H. Cardiac MRI findings in COVID-19 vaccine-related myocarditis: A pooled analysis of 468 patients. J Magn Reson Imaging. 2022;56:971–82.
Haj Mohamad Ebrahim Ketabforoush A, Molaverdi G, Nirouei M, Abbasi Khoshsirat N. Cerebral venous sinus thrombosis following intracerebral hemorrhage after COVID-19 AstraZeneca vaccination: A case report. Clin Case Rep. 2022;10:e6505.
Taga A, Lauria G. COVID-19 and the peripheral nervous system. A 2-year review from the pandemic to the vaccine era. J Peripher Nerv Syst JPNS. 2022;27:4–30.
Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43:3–40.
Watad A, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9:435.
Mirmosayyeb O, et al. Is myasthenia gravis a real complication of the COVID-19 vaccine? A case report-based systematic review. Can J Infect Dis Med Microbiol J Can Mal Infect Microbiol Medicale. 2022;2022:5009450.
Zargarbashi R, Vosoughi F, Milan N. Wide resection as a solution to excruciating pain in intraneural hemangioma: follow-up of a previously published case report. Int J Surg Case Rep. 2022;98:107562.
Tayebi AH, et al. Clinical features and outcomes of myasthenia gravis associated with COVID-19 vaccines: A systematic review and pooled analysis. Medicine (Baltimore). 2023;102:e34890.
Munn Z, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synthesis. 2019;18(10):2127–33.
Siddiqi AR, et al. Miller fisher syndrome after COVID-19 vaccination: case report and review of literature. Medicine (Baltimore). 2022;101:e29333.
Thant HL, et al. Guillain-Barré syndrome after Ad26.COV2.S vaccination. Am J Case Rep. 2022;23:e935275.
Masuccio FG, Comi C, Solaro C. Guillain-Barrè syndrome following COVID-19 vaccine mRNA-1273: a case report. Acta Neurol Belg. 2022;122:1369–71.
Finsterer J. Exacerbating Guillain–Barré Syndrome Eight Days after Vector-Based COVID-19 Vaccination. Case Rep Infect Dis. 2021;2021:1–3.
Abičić A, Adamec I, Habek M. Miller fisher syndrome following Pfizer COVID-19 vaccine. Neurol Sci. 2022;43:1495–7.
Matarneh AS, Al-battah AH, Farooqui K, Ghamoodi M, Alhatou M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin Case Rep. 2021;9:e04756.
Khadka B, Khanal K. Post COVID-19 vaccine Guillain-Barré syndrome. J Nepal Health Res Counc. 2022;19:852–4.
McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14:e244125.
Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: Temporal Associations Do Not Imply Causality. Neurology. 2021;96:1052–4.
Azam S, Khalil A, Taha A. Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca). Am J Med Case Rep. 2021;9:424–7.
Zubair AS, Bae JY, Desai K. Facial Diplegia variant of Guillain-Barré syndrome in pregnancy following COVID-19 vaccination: A case report. Cureus. 2022;14:e22341.
Michaelson NM, Lam T, Malhotra A, Schiff ND, MacGowan DJL. Miller fisher syndrome presenting after a second dose of Pfizer-BioNTech vaccination in a patient with resolved COVID-19: A case report. J Clin Neuromuscul Dis. 2021;23:113–5.
Hwang BW, Bong JB. Two possible etiologies of Guillain-Barré syndrome: mRNA-1273 (Moderna) vaccination and scrub typhus: A case report. Medicine (Baltimore). 2022;101:e32140.
Bouattour N, et al. Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Neurol Sci. 2022;43:755–61.
Prasad A, et al. A novel case of bifacial Diplegia variant of Guillain-Barré syndrome following Janssen COVID-19 vaccination. Neurol Int. 2021;13:404–9.
Morehouse ZP, Paulus A, Jasti SA, Bing X. A rare variant of Guillain-Barre syndrome following Ad26.COV2.S vaccination. Cureus. 2021;13:e18153.
Ilyas U, Umar Z, Bhangal R, Shah D, Fayman B. Guillain-Barré syndrome: A sequela of the original COVID-19 infection or vaccination. Cureus. 2022;14:e28044.
Dang YL, Bryson A. Miller-fisher syndrome and Guillain-Barre syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. 2021;14:e246701.
Nasuelli NA, et al. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol Sci. 2021;42:4747–9.
da Silva GF, et al. Guillain-Barré syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin Exp Neuroimmunol. 2022;13:92–4.
Rossetti A, Gheihman G, O’Hare M, Kosowsky JM. Guillain-Barré syndrome presenting as facial Diplegia after COVID-19 vaccination: A case report. J Emerg Med. 2021;61:e141–5.
Nishiguchi Y, Matsuyama H, Maeda K, Shindo A, Tomimoto H. Miller fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. BMC Neurol. 2021;21:452.
Scendoni R, Petrelli C, Scaloni G, Logullo FO. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum Vaccines Immunother. 2021;17:4093–6.
Ogbebor O, Seth H, Min Z, Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: A temporal occurrence, not a causal association. IDCases. 2021;24:e01143.
Kripalani Y, et al. A rare case of Guillain-Barré syndrome following COVID-19 vaccination. Eur J Case RepIntern Med. 2021;8:002707.
Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14:e243629.
Trimboli M, Zoleo P, Arabia G, Gambardella A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol Sci. 2021;42:4401–2.
Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14:e242956.
Lanman TA, Wu C, Cheung H, Goyal N, Greene M. Guillain-Barré syndrome with rapid onset and autonomic dysfunction following first dose of Pfizer-BioNTech COVID-19 vaccine: A case report. The Neurohospitalist. 2022;12:388–90.
Hughes DL, et al. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Liver Transpl. 2022;28:134–7.
Tutar NK, Eyigürbüz T, Yildirim Z, Kale N. A variant of Guillain-Barre syndrome after SARS-CoV-2 vaccination: AMSAN. Ideggyogyaszati Szle. 2021;74:286–8.
Nagalli S, Shankar Kikkeri N. Sub-acute onset of Guillain-Barré syndrome post-mRNA-1273 vaccination: a case report. SN Compr Clin Med. 2022;4:41.
Introna A, et al. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association? Clin Neurol Neurosurg. 2021;208:106887.
Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426.
Donaldson L, Margolin E. Variant Guillain-Barre syndrome following SARS-CoV-2 vaccination: case report and review of the literature. Can J Neurol Sci J Can Sci Neurol. 2022;1–3 https://doi.org/10.1017/cjn.2021.492.
Nanatsue K, Takahashi M, Itaya S, Abe K, Inaba A. A case of miller fisher syndrome with delayed onset peripheral facial nerve palsy after COVID-19 vaccination: a case report. BMC Neurol. 2022;22:309.
Jain E, Pandav K, Regmi P, Michel G, Altshuler I. Facial Diplegia: A rare, atypical variant of Guillain-Barré syndrome and Ad26.COV2.S vaccine. Cureus. 2021; https://doi.org/10.7759/cureus.16612.
Anjum Z, et al. Guillain-Barré syndrome after mRNA-1273 (Moderna) COVID-19 vaccination: A case report. Clin Case Rep. 2022;10:e05733.
Gunawan PY, Tiffani P, Lalisang L. Guillain-Barre syndrome following SARS-CoV-2 vaccination: A case report. Clin Psychopharmacol Neurosci. 2022;20:777–80.
Ogata S, et al. Sensory ataxic Guillain-Barré syndrome with Dysgeusia after mRNA COVID-19 vaccination. Intern Med Tokyo Jpn. 2022;61:1757–60.
Ling L, Bagshaw SM, Villeneuve P-M. Guillain-Barré syndrome after SARS-CoV-2 vaccination in a patient with previous vaccine-associated Guillain-Barré syndrome. CMAJ Can Med Assoc J J Assoc Medicale Can. 2021;193:E1766–9.
Bazrafshan H, Mohamadi Jahromi LS, Parvin R, Ashraf A. A case of Guillain-Barre syndrome after the second dose of AstraZeneca COVID-19 vaccination. Turk J Phys Med Rehabil. 2022;68:295–9.
Hilts A, Schreiber A, Singh A. A clinical case of COVID-19 vaccine-associated Guillain-Barré syndrome. Am J Case Rep. 2022;23:e936896.
Pirola FJC, et al. Miller-fisher syndrome after first dose of Oxford/AstraZeneca coronavirus disease 2019 vaccine: a case report. J Med Case Rep. 2022;16:437.
Bellucci M, et al. Case report: post-COVID-19 vaccine recurrence of Guillain-Barré syndrome following an antecedent Parainfectious COVID-19-related GBS. Front Immunol. 2022;13:894872.
Rao SJ, et al. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J Community Hosp Intern Med Perspect. 2021;11:597–600.
Malamud E, Otallah SI, Caress JB, Lapid DJ. Guillain-Barré syndrome after COVID-19 vaccination in an adolescent. Pediatr Neurol. 2022;126:9–10.
Prado MB, Adiao KJB. Facial Diplegia as the sole manifestation of post-vaccination Guillain-Barre syndrome: A case report and literature review. The Neurohospitalist. 2022;12:508–11.
Richardson-May J, Purcaru E, Campbell C, Hillier C, Parkin B. Guillain-Barré syndrome and unilateral optic neuritis following vaccination for COVID-19: A case report and literature review. Neuro-Ophthalmol Aeolus Press. 2022;46:413–9.
Aomar-Millán IF, Martínez de Victoria-Carazo J, Peregrina-Rivas JA, Villegas-Rodríguez I. COVID-19, Guillain-Barré y vacuna. Una mezcla peligrosa. Rev Clínica Esp. 2021;221:555–7.
Čenščák D, Ungermann L, Štětkářová I, Ehler E. Guillan-Barré syndrome after first vaccination dose against COVID-19: case report. Acta Med (Hradec Kralove). 2021;64:183–6.
Kim N, Kim J-H, Park J-S. Guillain-Barré syndrome associated with BNT162b2 COVID vaccination: a first case report from South Korea. Neurol Sci. 2022;43:1491–3.
Kim Y, et al. A pediatric case of sensory predominant Guillain-Barré syndrome following COVID-19 vaccination. Child Neurol Open. 2022;9:2329048X221074549.
Finsterer J. Guillain-Barre syndrome 15 days after COVID-19 despite SARS-CoV-2 vaccination. IDCases. 2021;25:e01226.
Theuriet J, et al. Guillain-Barré syndrome following first injection of ChAdOx1 nCoV-19 vaccine: first report. Rev Neurol (Paris). 2021;177:1305–7.
Liang H, et al. Miller-fisher syndrome and Guillain-Barre syndrome overlap syndrome following inactivated COVID-19 vaccine: case report and scope review. Hum Vaccines Immunother. 2022;18:2125753.
Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann Med Surg. 2021;67:102540.
Allen CM, et al. Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90:315–8.
Bax F, Gigli GL, Belgrado E, Brunelli L, Valente M. Guillain-Barré syndrome following Covid-19 immunization: a report of two cases. Acta Neurol Belg. 2022;122:1365–7.
Bonifacio GB, et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2022;93:341–2.
Min YG, et al. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: report of two cases and review of literature. J Neuroimmunol. 2021;359:577691.
Germano F, et al. COVID-19 vaccine-related Guillain-Barré syndrome in the Liguria region of Italy: A multicenter case series. J Neurol Sci. 2022;440:120330.
Hai PD, et al. Guillain-Barré syndrome after COVID-19 vaccination: report of two cases from Vietnam. J Infect Dev Ctries. 2022;16:1703–5.
Berrim K, et al. Guillain-Barré syndrome after COVID-19 vaccines: A Tunisian case series. Br J Clin Pharmacol. 2023;89:574–8.
James J, Johnson J, Jose J. Neuralgic Amyotrophy after ChAdOx1 nCoV-19 COVID-19 vaccination. J Clin Neuromuscul Dis. 2022;24:112–3.
Kanabar G, Wilkinson P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021;14:e244527.
Karimi N, et al. Guillain-Barre syndrome and COVID-19 vaccine: A report of nine patients. Basic Clin Neurosci. 2021;12:703–10.
Chun JY, et al. Guillain-Barré syndrome after vaccination against COVID-19. Lancet Neurol. 2022;21:117–9.
Castiglione JI, et al. Bilateral facial palsy with paresthesias, variant of Guillain-Barré syndrome following COVID-19 vaccine: A case series of 9 patients. Neuromuscul Disord NMD. 2022;32:572–4.
Nagdev G, Chavan G, Sahu G, Devasilpa Raju PD. COVID-19 vaccination a cause of Guillain-Barré syndrome? A Case Series. Cureus. 2022;14:e30888.
Tabatabaee S, et al. Post COVID-19 vaccination Guillain-Barre syndrome: three cases. Hum Vaccines Immunother. 2022;18:2045153.
Wan MM, Lee A, Kapadia R, Hahn C. Case series of Guillain-Barré syndrome after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. Neurol Clin Pract. 2022;12:149–53.
Oo WM, Giri P, de Souza A. AstraZeneca COVID-19 vaccine and Guillain- Barré syndrome in Tasmania: A causal link? J Neuroimmunol. 2021;360:577719.
García-Grimshaw M, et al. Guillain-Barré syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol. 2021;230:108818.
Kim J-E, et al. Guillain-Barré syndrome and variants following COVID-19 vaccination: report of 13 cases. Front Neurol. 2021;12:820723.
Kim JW, et al. Guillain-Barre syndrome after two COVID-19 vaccinations: two case reports with follow-up Electrodiagnostic study. J Korean Med Sci. 2022;37:e58.
Osowicki J, et al. Guillain-Barré syndrome in an Australian state using both mRNA and adenovirus-vector SARS-CoV −2 vaccines. Ann Neurol. 2021;90:856–8.
Maramattom BV, et al. Guillain-Barré syndrome following ChAdOx1-S / nCoV −19 vaccine. Ann Neurol. 2021;90:312–4.
Shields LBE, Iyer VG, Zhang YP, Burger JT, Shields CB. Parsonage-turner syndrome following COVID-19 vaccination: clinical and Electromyographic findings in 6 patients. Case Rep Neurol. 2022;14:58–67.
Queler SC, Towbin AJ, Milani C, Whang J, Sneag DB. Parsonage-turner syndrome following COVID-19 vaccination: MR Neurography. Radiology. 2022;302:84–7.
Min YG, et al. Parsonage-turner syndrome following COVID-19 vaccination. J Neurol Neurosurg Psychiatry. 2022;93:1231–2.
Koh JS, et al. Neuralgic amyotrophy following COVID-19 mRNA vaccination. QJM Int J Med. 2021;114:503–5.
Vitturi BK, et al. Parsonage-turner syndrome following coronavirus disease 2019 immunization with ChAdOx1-S vaccine: a case report and review of the literature. J Med Case Rep. 2021;15:589.
Sharma R, Dua B, Goyal S, Tiwari T. Parsonage–Turner Syndrome Following COVID-19 Vaccine. Ann Indian Acad Neurol. 2022;25:973–5.
Öncel A, Coşkun E. Parsonage-turner syndrome after SARS-CoV-2 vaccination: A case report. Turk J Phys Med Rehabil. 2022;68:418–21.
Mejri I, et al. Parsonage-turner syndrome of the brachial plexus secondary to COVID-19 vaccine: A case report. Clin Case Rep. 2022;10:e6483.
Mahajan S, Zhang F, Mahajan A, Zimnowodzki S. Parsonage turner syndrome after COVID-19 vaccination. Muscle Nerve. 2021;64:E3–4.
Lakkireddy M, Sathu S, Kumar R, Madhu Latha K, Maley DK. Parsonage-turner syndrome following Covishield (AstraZeneca ChAdOx1 nCoV-19) vaccination: A case report. Cureus. 2022;14:e27867.
Fukahori K, et al. Neuralgic Amyotrophy after COVID-19 vaccination in an adolescent: successful intravenous immunoglobulin treatment. Pediatr Neurol. 2022;140:50–1.
Amjad MA, et al. COVID-19 vaccine-induced parsonage-turner syndrome: A case report and literature review. Cureus. 2022;14:e25493.
Bernheimer JH, Gasbarro G. Parsonage turner syndrome following vaccination with mRNA-1273 SARS-CoV-2 vaccine. J Clin Neuromuscul Dis. 2022;23:229–30.
Civardi C, Delconte C, Pisano F, Collini A, Geda C. Isolated musculocutaneous involvement in neuralgic amyotrophy associated with SARS-CoV2 vaccination. Neurol Sci. 2022;43:3515–7.
Diaz-Segarra N, et al. Painless idiopathic neuralgic amyotrophy after COVID-19 vaccination: A case report. PM R. 2022;14:889–91.
Crespo Burillo JA, Loriente Martínez C, García Arguedas C, Mora Pueyo FJ. Amyotrophic neuralgia secondary to Vaxzevri (AstraZeneca) COVID-19 vaccine. Neurol Engl Ed. 2021;36:571–2.
Coffman JR, Randolph AC, Somerson JS. Parsonage-Turner Syndrome After SARS-CoV-2 BNT162b2 Vaccine: A Case Report. JBJS Case Connector. 2021;11(3):e21.00370. https://doi.org/10.2106/JBJS.CC.21.00370.
Flikkema K, Brossy K. Parsonage-Turner Syndrome After COVID-19 Vaccination: A Case Report. JBJS Case Connect. 2021;11(4):e21.00577. https://doi.org/10.2106/JBJS.CC.21.00577.
Kim SI, Seok HY, Yi J, Cho JH. Leg paralysis after AstraZeneca COVID-19 vaccination diagnosed as neuralgic amyotrophy of the lumbosacral plexus: a case report. J Int Med Res. 2021;49:030006052110567.
Chua MMJ, Hayes MT, Cosgrove R. Parsonage-turner syndrome following COVID-19 vaccination and review of the literature. Surg Neurol Int. 2022;13:152.
Mirmosayyeb O, Barzegar M, Rezaei M, Baharlouie N, Shaygannejad V. Bell’s palsy after sputnik V COVID-19 (gam-COVID-Vac) vaccination. Clin Case Rep. 2022;10:e05468.
SALBAŞ E, ÖCEK Ö, ÖCEK L, KARAHAN AY. Letter to the Editor: Facial paralysis following messenger RNA COVID-19 Vaccines: The report of two Cases. Ege Tıp Bilimleri Dergisi. 2021;4(2):73–7.
Repajic M, Lai XL, Xu P, Liu A. Bell’s palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun – Health. 2021;13:100217.
Poudel S, Nepali P, Baniya S, Shah S, Bogati S, Nepal G, Ojha R, Edaki O, Lazovic G, Kara S. Bell's palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. https://doi.org/10.1016/j.amsu.2022.103897.
Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol. 2021;268:3589–91.
Pothiawala S. Bell’s palsy after second dose of Moderna COVID-19 vaccine: coincidence or causation? Acta Medica Litu. 2021;28:298–301.
Iftikhar H, Noor SMU, Masood M, Bashir K. Bell’s palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus. 2021;13:e15935.
Zhang H, Sanchez Gomez D, Repajic M, Liu AK. Another case of Bell’s palsy recurrence after Pfizer-BioNTech COVID-19 vaccination. Cureus. 2022;14:e27422.
Nishizawa Y, Hoshina Y, Baker V. Bell’s palsy following the Ad26.COV2.S COVID-19 vaccination. QJM Int J Med. 2021;114(9):657–8. https://doi.org/10.1093/qjmed/hcab143.
Cellina M, D’Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell’s palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin Imaging. 2022;82:1–4.
Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2022;269:47–8.
Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42:4397–9.
Yu B-Y, Cen L-S, Chen T, Yang T-H. Bell’s palsy after inactivated COVID-19 vaccination in a patient with history of recurrent Bell’s palsy: A case report. World J Clin Cases. 2021;9:8274–9.
Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14:e243829.
Ish S, Ish P. Isolated peripheral facial nerve palsy post COVID-19 vaccination with complete clinical recovery. Indian J Ophthalmol. 2022;70:347.
Galimi R, Galimi M. Adverse Event Reporting after mRNA COVID-19 Vaccination: A Bell’s Palsy Case the Day after. Neurol Case Rep. 2021;4(2):1026.
Mussatto CC, Sokol J, Alapati N. Bell’s palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Rep. 2022;25:101259.
Khokhar F, Khan A, Hussain Z, Yu J. Small Fiber neuropathy associated with the Moderna SARS-CoV-2 vaccine. Cureus. 2022;14:e25969.
Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64:E1–2.
Finsterer J. Small fiber neuropathy as a complication of SARS-CoV-2 vaccinations. J Fam Med Prim Care. 2022;11:4071.
Chuang TY, Burda K, Teklemariam E, Athar K. Tolosa-hunt syndrome presenting after COVID-19 vaccination. Cureus. 2021;13:e16791.
Fanella G, et al. New-onset myasthenia gravis after mRNA SARS-CoV-2 vaccination: a case series. Neurol Sci. 2022;43:5799–802.
Ramdas S, et al. SARS-CoV-2 vaccination and new-onset myasthenia gravis: A report of 7 cases and review of the literature. Neuromuscul Disord. 2022;S0960896622006502 https://doi.org/10.1016/j.nmd.2022.09.001.
Devaraj R, Shafi P, Nagesh C, Naidu A, Satishchandra P. Spectrum of neurological complications following COVID-19 vaccination in India. J Clin Neurol Seoul Korea. 2022;18:681–91.
Abicic A, Sitas B, Adamec I, Bilic E, Habek M. New-onset ocular myasthenia gravis after booster dose of COVID-19 vaccine. Cureus. 2022; https://doi.org/10.7759/cureus.27213.
Chavez A, Pougnier C. A case of COVID-19 vaccine associated new diagnosis myasthenia gravis. J Prim Care Community Health. 2021;12:215013272110519.
Hoshina Y, Sowers C, Baker V. Myasthenia gravis presenting after administration of the mRNA-1273 vaccine. Eur J Case Rep Intern Med. 2022;1 https://doi.org/10.12890/2022_003439.
Kang MC, Park K-A, Min J-H, Oh SY. Myasthenia gravis with ocular symptoms following a ChAdOx1 nCoV-19 vaccination: A case report. Am J Ophthalmol Case Rep. 2022;27:101620.
Lee MA, Lee C, Park JH, Lee JH. Early-onset myasthenia gravis following COVID-19 vaccination. J Korean Med Sci. 2022;37:e50.
Slavin E, Fitzig J, Neubert C, Garcia-Lopez F, Cuevas-Trisan R. New-Onset Myasthenia Gravis Confirmed by Electrodiagnostic Studies After a Third Dose of SARS-CoV-2 mRNA-1273 Vaccine. Am J Phys Med Rehabil. 2022;101(12):e176-9. https://doi.org/10.1097/PHM.0000000000002076.
Maher DI, Hogarty D, Ben Artsi E. Acute onset ocular myasthenia gravis after vaccination with the Oxford-AstraZeneca COVID-19 vaccine. Orbit. 2022;1–5 https://doi.org/10.1080/01676830.2022.2062777.
Galassi G, Marchioni A. Myasthenia gravis at the crossroad of COVID-19: focus on immunological and respiratory interplay. Acta Neurol Belg. 2021;121:633–42.
Virgilio E, Tondo G, Montabone C, Comi C. COVID-19 vaccination and late-onset myasthenia gravis: A new case report and review of the literature. Int J Environ Res Public Health. 2022;20:467.
Davalos L, Kushlaf H. New onset of seropositive generalized myasthenia gravis following intravesical bacille Calmette-Guerin treatment for bladder cancer: A case study. Muscle Nerve. 2019;59:E1–2.
Chung JY, Lee SJ, Shin B-S, Kang HG. Myasthenia gravis following human papillomavirus vaccination: a case report. BMC Neurol. 2018;18:222.
Takizawa T, et al. New onset of myasthenia gravis after intravesical Bacillus Calmette-Guerin: A case report and literature review. Medicine (Baltimore). 2017;96:e8757.
Stübgen J-P. Neuromuscular disorders associated with hepatitis B vaccination. J Neurol Sci. 2010;292:1–4.
Wang F, et al. Population-based incidence of Guillain-Barré syndrome during mass immunization with viral vaccines: A pooled analysis. Front Immunol. 2022;13:782198.
Ahsanuddin S, Nasser W, Roy SC, Povolotskiy R, Paskhover B. Facial paralysis and vaccinations: a vaccine adverse event reporting system review. Fam Pract. 2022;39:80–4.
Sanghani N, Rajanigandhi H, Shreya S, Nizar S. “Myasthenia Gravis after Vaccination in Adults the United States: A Report from the CDC/FDA Vaccine Adverse Event Reporting System (1990–2017) (P6.437).” Neurology. 2018;90.
Abolmaali M, et al. Guillain-Barré syndrome in association with COVID-19 vaccination: a systematic review. Immunol Res. 2022;70:752–64.
Acknowledgments
Researchers appreciated the Clinical Research Development Units of Kamali and Rajaei Hospitals at Alborz University of Medical Sciences.
Funding
There is no funding source with authors to declare.
Author information
Authors and Affiliations
Contributions
A.T, P.S, and H.R contributed to the conception and design of the study. E.J organized the database. P.S performed the statistical analysis. A.T wrote the first draft of the manuscript. S.M, A.A, N.M, and A.M wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Quality assessment of the included case series based on the JBI checklist for case series. Supplementary Table 2. Guillain barre syndrome studies and patients’ characteristics by case. Supplementary Table 3. Guillain Barre patients’ findings, treatments, and outcomes by case. Supplementary Table 4. Parsonage turner studies and patients’ characteristics by case. Supplementary Table 5. Parsonage turner patients’ findings, treatments, and outcomes by case. Supplementary table 6. Facial nerve palsy patients’ findings, treatments, and clinical outcomes by case. Supplementary table 7. Small fiber neuropathy and tolosa-hunt patients’ findings, treatments, and clinical outcomes by case. Supplementary Table 8. Myasthenia gravis studies and patients’ characteristics by case. Supplementary Table 9. Myasthenia gravis patients’ findings, treatments, and clinical outcomes by case.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tayebi, A., Samimisedeh, P., Jafari Afshar, E. et al. Neuromuscular diseases associated with COVID-19 vaccines: a systematic review and pooled analysis of 258 patients. BMC Neurol 23, 437 (2023). https://doi.org/10.1186/s12883-023-03486-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03486-y